CSSD Services
Zehnacker’s off-site CSSD delivers high-quality and compliant sterilisation services you can rely on. Our solutions help healthcare facilities ensure safe, compliant sterilisation while reducing infection risks and improving efficiency.

OVERVIEW
why OFF-SITE REPROCESSING
Zehnacker provides a fully managed sterilisation service designed to integrate seamlessly with your surgical workflow.
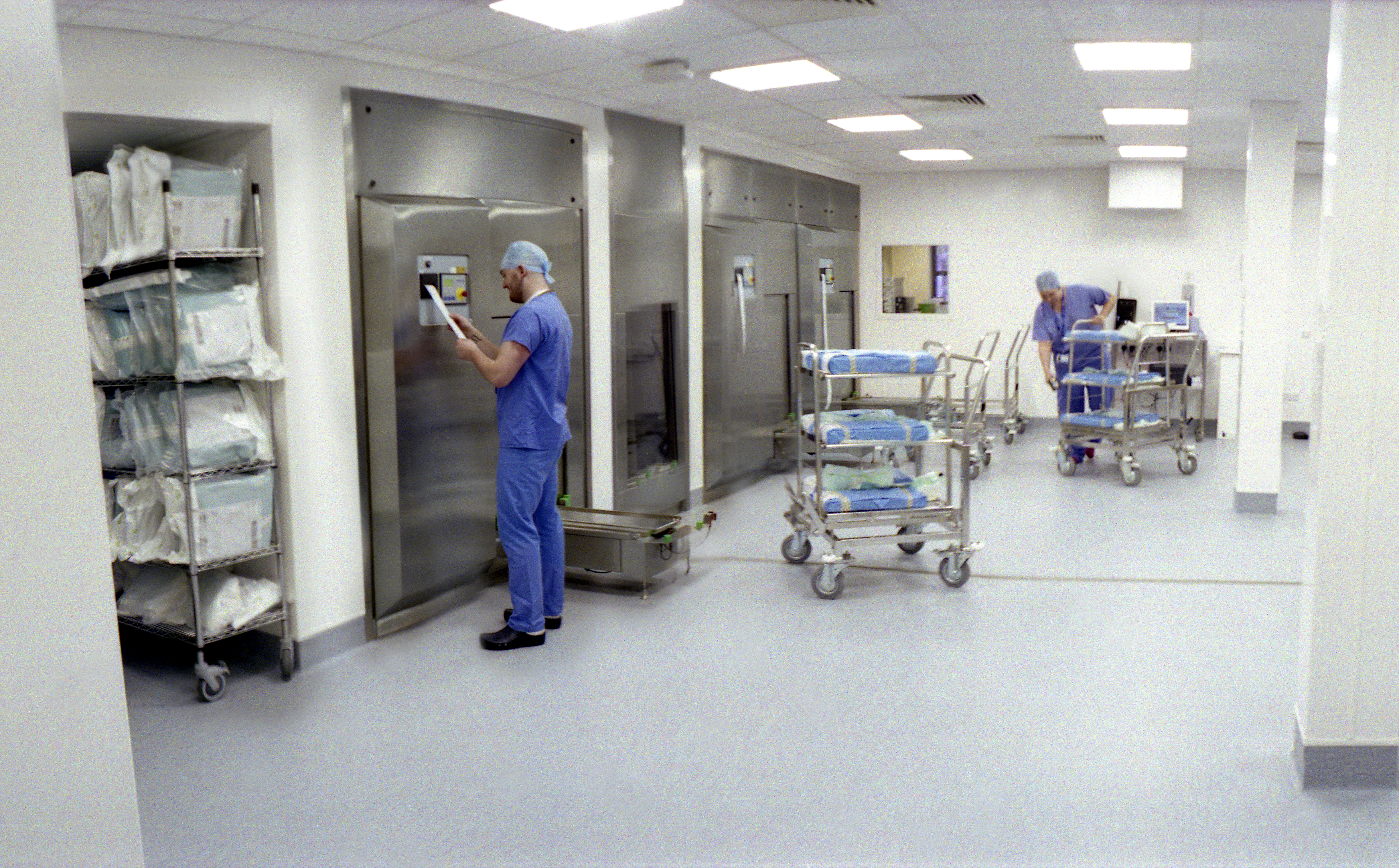
Established in 2014, our off-site CSSD is one of Ireland’s most advanced units for the reprocessing of Reusable Invasive Medical Devices (RIMDs), delivering consistent turnaround and full traceability in ISO 13485 / ISO 9001 certified facilities.
Benefits for Your Hospital:
- Ensures continuity of surgical services when on-site capacity is limited
- Frees up internal space for clinical or revenue-generating services
- Reduces risk of non-compliance
- Removes the need for capital investment in facilities, equipment or staffing
- Delivers predictable costs and reduces operational risk
- Provides access to trained, experienced CSSD technicians and modern equipment
- Supports hospitals in meeting increasing surgical volumes
SOLVING CSSD BOTTLENECKS
When Off-Site Reprocessing Makes Sense
Whether you’re scaling up, short on space, or facing staffing challenges, off-site reprocessing provides a reliable, flexible solution that keeps surgical services running without disruption. It’s ideal if:
- Your on-site CSSD is under pressure or facing space constraints
- You’re planning renovations, relocation, or infrastructure upgrades
- You need to repurpose internal space for clinical care or income-generating activity.
- You’re struggling to recruit or retain trained decontamination staff
- You’re experiencing recurring tray delays, backlogs, or loan set issues
- You need a reliable contingency plan for service interruptions
- You’re finding it more and more difficult to keep up with complex IFUs
- You want to standardise quality, traceability and compliance across multiple sites
- You want to reduce the risk related to existing old or non compliant infrastructure without capital expenditure

end to end assurance
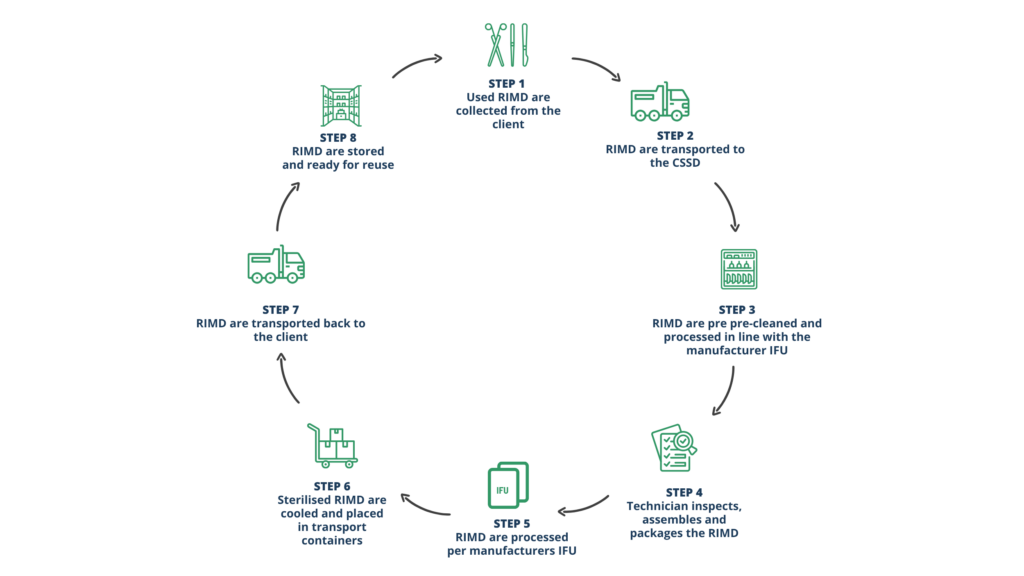
OUR PROCESS
Collection
Processing
Delivery
- RIMDs are collected from a designated point at the client site.
- We supply ADR 2023-compliant containers and dedicated vehicles.
- Transport containers are decontaminated after each use in a validated trolley washer.
- Items are logged using our Fingerprint electronic traceability system, ensuring end-to-end tracking.
- Each device is disassembled, visually inspected and manually cleaned.
- Ultrasonic cleaning is available as required
- Six washer-disinfectors with specialised lumen ports support high-throughput processing.
- Sterilisation is completed in steam autoclaves or Hyper LTS 150-2 for low-temperature needs.
- Processing takes place in ISO 14644-certified cleanroom environments.
- All activity follows strict SOPs aligned with ISO 13485 and manufacturer IFU
- Delivery is completed on a fixed and agreed schedule.
- Sterile RIMDs are packed in single-use sealed bags and transported in solid boxes.
- Strict segregation of used and sterile RIMD during transport
- All transport containers are reprocessed after each use.
- ADR 2023 compliant transport systems
- Dedicated vehicles with cleanable loading areas
- Thermal disinfection of transport boxes after each use
- Full audit trail provided with itemised delivery documentation.
CERTIFIED QUALITY BUILT-IN
Quality
Zehnacker delivers sterilisation services under a fully audited, certified framework built for regulatory confidence and clinical assurance. Every process, from washer validation to pack release, is governed by international standards and hospital-specific oversight.
At a Glance:
- Full audit trail to cycle records
- ISO 13485 / ISO 9001 certified facility
- Compliance with EU MDR
- Instrument-Level Inspection & Function Testing
- Dedicated Quality Lead
- Validated washer-disinfectors and decontamination workflows
- Independent AED audited process


SPECIALIST LOGISTICS, BUILT FOR CSSD
LOGISTICS & TRANSPORT
When every minute counts and compliance is non-negotiable, we simply deliver.
Transport is fully integrated into the reprocessing model so that every movement is documented, traceable and accountable.
We provide a validated logistics service built specifically for sterile services, with strict separation of clean and contaminated flows, timed collections and emergency response capability.
We Deliver:
- Full segregation of clean and dirty instruments
- Scheduled collections aligned to hospital workflows
- Emergency logistics support 7 days a week
- Documented chain of custody for every journey
- Nationwide logistics service
SAFE & COMPLIANT
traceability
Zehnacker utlises the Fingerprint system which provides full visibility across the entire reprocessing workflow, from initial collection through to validated return.
Barcode tracking is integrated at every control point. Cycle records and load reports are stored digitally and can be accessed instantly for audit, clinical governance, or reporting purposes.
Our system includes:
- Full compliance with ISO recordkeeping requirements
- Instrument and tray-level tracking throughout the process
- Barcode scan points at each stage of the workflow

GET IN TOUCH
CONTACT US
We want to hear from you. If you have any questions or queries, one of our team members is here to help.

